Which would you choose? Diamonds or banana peels? Diamonds, right?

Well, maybe this needs rethinking.
I’ve been researching for my book for Nomad Press, all about chemical reactions! Chemistry is cool. And I love research. It’s amazing what a single element can do—if it’s the right element. On our planet, one of those elements is carbon!


All life on Earth is carbon-based. Bacteria, plants, animals, and people. Humans are 60 percent water, but if you take apart a 150-pound adult, 35 pounds of him or her is carbon. People who are totally immersed in this topic study organic chemistry, which is the chemistry of carbon compounds. Sugars, proteins, fats, DNA, all qualify.
Yes, I would choose sugar! Specifically chocolate.

DIAMOND FACTS
Diamonds are one of carbon’s different forms.

Louie isn’t envious of the diamond choker on the Chihuahua. He’s happy with popcorn!
Most diamonds are formed by massive temperatures of 1050 degrees Celsius (1200 degrees Fahrenheit) and pressures acting upon carbon-containing minerals in the earth’s mantle layer at about 1100 kilometers below the surface (60 miles down)
To find new pockets of diamonds, some geologists focus on the junction between the core and mantle. It’s complicated, and finding locations with diamonds requires information from plate tectonics, typically studied by seismologists and geologists.
As a point of reference, coal –another form of carbon—can form at 4 kilometers below the earth’s surface.
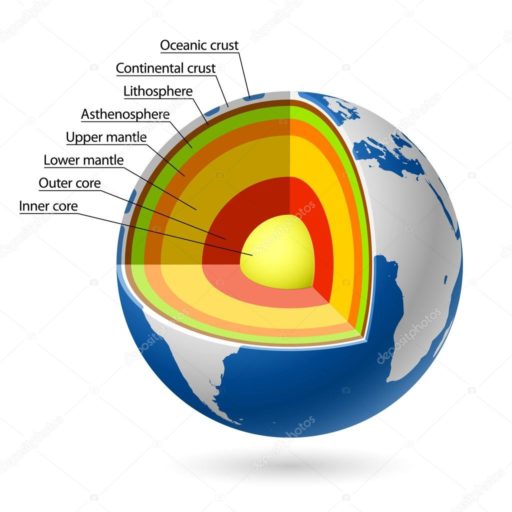
The diamonds for commercial use, aka the ones that end up in necklaces and rings, often come closer to the earth’s surface through volcanic eruptions.
(Hawaii lava photo courtesy Carolyn Holton)
Another common form of pure carbon is graphite, but it’s softer and not as gorgeous as a diamond. This is because of the structure/shape of the molecule, or how the atoms bond together. These different forms of the same element are called allotropes. Graphite is used in pencils.
Carbon has the most allotropes of any element.
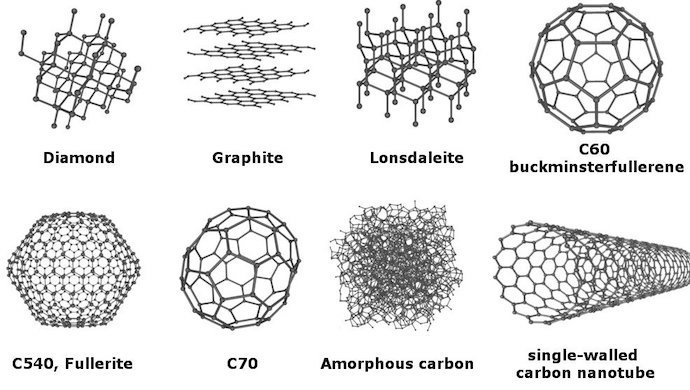
How many allotropes does carbon have? Eight!
Point of reference, oxygen has two allotropes, oxygen and ozone.
At very high temperatures, diamonds can even be converted to graphite. What do you think we should do with the people who spent time on that experiment ?
GRAPHENE
Graphene is another allotrope of carbon. (…remember this is a fancy term for a different structure of the same element)
Graphene is amazing! It’s one atom thin, flexible, nearly transparent, and 100 times stronger than steel. It’s got superb electrical and thermal conductivity. Bad news is that it’s expensive.
BANANA PEELS
In 2010, a Nobel prize in physics was awarded jointly because of ‘groundbreaking experiments’ regarding graphene! Scientific breakthroughs keep rolling out. Graphene has been shown to enhance touch screens, semiconductors, batteries and solar cells, to name a few. Thousands of patents are being filed every year for inventions ranging from graphene tires to flexible cellphones.

Let’s get back to the banana peels!
Chemists at Rice University in Texas can take any carbon material and turn it into graphene!
They’d like to see graphene put into concrete, which would increase its strength by 35% while decreasing the weight of the blocks. Asphalt and other building materials could benefit, to create stronger, longer lasting products. These applications would require literal tons of graphene. Right now, this is still cost prohibitive.
To produce graphene with this new method, the carbon material needs to be heated to 3000 Kelvin (5000 degrees Fahrenheit).
The great news is that food waste, plastic waste, dead insects, chocolate… even banana peels would work!
This method is called flash graphene. It would bring down the cost of graphene, revolutionizing industries. Especially with inexpensive raw materials…like bananas!
Cost was difficult to for me pinpoint and it does fluctuate, but currently, high-quality graphene runs about $250 for a two-inch square. Remember, this square is one atom thick. It doesn’t weigh much. So 10 ounces of bananas could turn a $15 billion profit!
As one of my friends says, with that much $ to do good deeds, we could be, uh, pooping rainbows.

So what do you think? Diamonds or bananas?














Another excellent science blog @susanberkkoch.com
You make me wonder about things I have never wondered about before…
That’s what I love about science! All the wondering. Thanks for stopping by!
Loved your article, think I still prefer diamonds
Thank you. And I definitely see your point about diamonds…
Very entertaining! The stuff about concrete really interested me. Bananas all the way!
That’s what inspired me to write the post! I love the idea of using graphene in building materials. Go bananas.
Great content! Super high-quality! Keep it up! 🙂
Thank you!
I’m voting diamonds, but I’ll never look at bananas the same way. Great research!
Thanks for stopping by! Diamonds are more sparkly!
Wow, this post was amazing! And you proved again how amaizing our world is!
Thanks so much! Science is pretty amazing.
Hi i was telling my dauters, it takes a lot of bandanas to cut a diamonds, which was true, but now it seams. I may be wrong, once again.
Keep up the good work,
I am now hoing to explain how the water from your bike wheel travels faster then your bum.
Tim miled
Not sure about the speed of water and bike wheels and bums. Let me know what you come up with. Thank you.
Thank you for a great article.
You’re welcome. Graphene’s been in the news lately so I’m pleased that research is ongoing. Thank you.